LaunchWorks One-Step RT-qPCR Master Mix:
Sensitive, Reproducible Detection
The LaunchWorks One-Step RT-qPCR Master Mix was designed for sensitive and reproducible detection of RNA and/or DNA targets in a single or multiplex RT-qPCR reaction. The 2X format was developed to guarantee simple reaction setup and robust performance. The Master Mix has been formulated and is manufactured in LaunchWorks’ ISO 13485, cGMP compliant facility, ensuring stringent production and quality control measures.
LaunchWorks xNetic™ Viral DNA/RNA Extraction Kit
The LaunchWorks xNetic™ Viral DNA/RNA Extraction Kit allows for a one-step magnetic bead-based extraction and purification of Viral RNA in less than 15 minutes. The procedure, for manual processing, only requires a magnetic rack and RNase-free microfuge tubes. Our technology has been validated for a variety of commercially available automated platforms with results that exceed performance compared to industry standards. The LaunchWorks xNetic Viral DNA/RNA Extraction Kit is a cost-effective solution that requires less components, less time and less capital investment for diagnostic sample preparation.
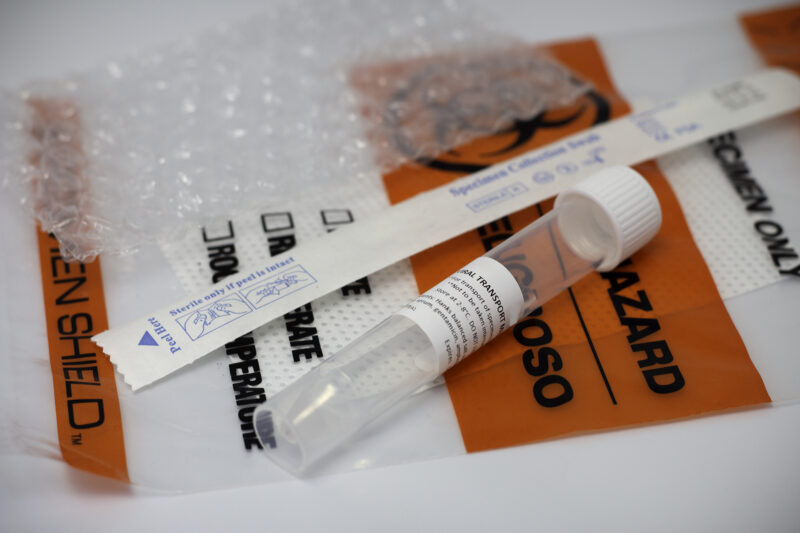
Viral Transport Media for COVID-19 Specimen collection
FDA Listed for EUA
The FDA has issued Emergency Use Authorizations (EUA) for numerous molecular and antigen tests for detection of SARS-CoV-2 (COVID-19). Our Viral Transport Media is listed per FDA guidance, “Enforcement Policy for Viral Transport Media during the Coronavirus 2019 (COVID-19) Public Health Emergency.”
The VTM is manufactured in the LaunchWorks CDMO facility which is FDA registered, cGMP compliant, and ISO 13485:2016 certified.